New Delhi: Prime Minister Narendra Modi in his address to the nation on Saturday (December 25) said that India will soon start administering the nasal vaccine and the world’s first DNA vaccine against COVID-19.
Amid the rising COVID-19 cases due to the Omicron type of virus, the PM appealed not to panic but to be alert and use masks and sanitize hands regularly.
The PM in his address also pointed out that vaccination against COVID-19 for 15-18-year-olds in the country will begin on January 3, while the “precautionary dosing” for health care and frontline workers will begin on January 10.
“People with comorbidities and above 60 years of age will be eligible for the precautionary dose from January 10, 2022 on the recommendation of their doctors,” the PM announced.
Here’s Nasal COVID-19 Vaccine Ready for January in India; Here’s how it will be a ‘game changer’
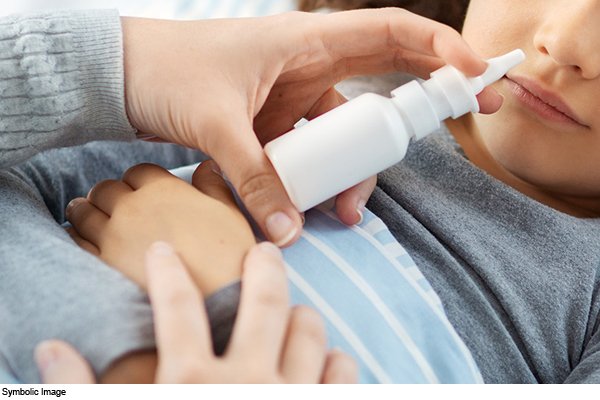
Three-dose nasal vaccine, the world’s first DNA vaccine by Zydus Cadila, to be available soon for India’s COVID-19 vaccination campaign.
The vaccine will be administered using a needle-free applicator, unlike conventional syringes.
The vaccine will be administered using a needle-free applicator as opposed to conventional syringes.
The announcement comes at a critical time when India is witnessing a sharp rise in the omicron version of COVID-19 and the World Health Organization has reiterated vaccination as the only way through a possible third wave.
What is a nasal vaccine
Nasal vaccine is administered through the nose and not syringe making it easy for application to children. It can generate an immune response at the site of infection within respiratory passage starting from nasal airways and is most suitable for children as it blocks both infection and transmission of Covid-19.
How the nasal vaccine will be administered
The vaccine will be administered using a needle-free applicator as opposed to the traditional syringes. The applicator is called “PharmaJet”.
PharmaJet is a needle-free applicator to ensure painless intradermal vaccine delivery, which also leads to a significant reduction in any kind of major side effects. The three doses of ZyCoV-D are to be administered 28 days apart.
Which other nasal vaccines are being developed in India?
Apart from Zydus Cadila, Bharat Biotech is developing a BBV154 nasal vaccine for children in collaboration with Washington University School of Medicine (WUSM). Bharat Biotech das applied for regulatory nod to conduct Phase-3 trials of its nasal Covid-19 vaccine that can be used as a booster shot for those vaccinated with Covaxin and Covishield.
Serum Institute of India (SII) , already administering a syringe based Covaxin and developing another for children is also testing the efficacy of the intransal COVI-VAC Covid vaccine in collaboration with New York-based vaccine maker Codagenix.
What WHO have to say about intranasal vaccines?
World Health Organisation’s Chief Scientist Dr Soumya Swaminathan has said that the nasal vaccine could be a ‘game-changer for children’ as they are easy to administer and will give local immunity at respiratory tract.

