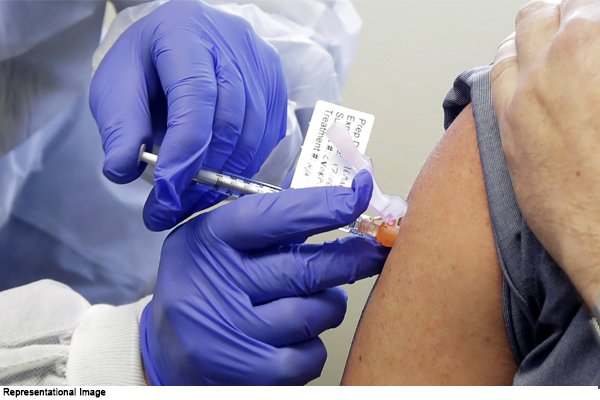
New Delhi: The dry run of the Covid-19 vaccine is scheduled to begin across India from today. Through this dry run, you will know how to deliver this vaccine to people and how to use it.

Also know about its loopholes. A day after the massive exercise, a panel of experts appointed by the government has recommended approval for the Oxford covid-19 vaccine produced by the Serum Institute of India to the Regulatory Drug Control Authority of India. Today’s exercise is the country’s second dry run – the first was done on December 28 and December 29 in Assam, Andhra Pradesh, Punjab and Gujarat.
The dry run of the vaccine will be started today after the emergency use of the corona vaccine has been approved. The actual vaccination campaign will be carried out on the basis of this dry run to be conducted across the country. Explain that no vaccine will be used during the dry run. Through this, only this test will be done on how effective the government has prepared the vaccination plan.
All over India, this dry run is being run in 259 places in 116 districts. The Health Ministry had said that some 96,000 vaccinators have been trained for this. Of these, more than 57,000 people have been trained with 2,360 participants national training of trainers and district-level training in 719 districts.
In this process, 25 health workers will be present with dummy vaccines at each location. “The special focus in the dry run will be on the management of any potential adverse events after vaccination,” the Health Ministry said in a statement.
Health Minister Harsh Vardhan has asked the officials in all the states to ensure that the vaccination site and in-charge follow the checklist and standard operating procedure prepared by the Ministry of Health and share it with all states and union territories and allow them to run this dry run. Guide in
Health Minister will supervise the dry run in the national capital. The site is Guru Tegh Bahadur Hospital in Shahdara, Urban Primary Health Center in Daryaganj and Venkateswara Hospital in Dwarka.
In Lucknow, a dry run will be conducted at six locations. In Pune, it will be held at three health centers besides Nagpur, Jalna and Nandurbar. Seven districts will be dry in Chhattisgarh. One day campaign will be conducted in four districts in Gujarat. Punjab will conduct a dry run in Patiala, and Haryana will practice in Panchkula. In Kerala, the drought run will be conducted in four districts – Thiruvananthapuram, Idukki, Wayanad and Palakkad.
India is awaiting vaccination for Covid-19 and it will start soon when the regulator DCGI approves a vaccine. The Serum Institute of India is developing a vaccine covicil developed by Oxford University and pharma major AstraZeneca, while Bharat Biotech has partnered with the Indian Council of Medical Research (ICMR) for its Covaxin.
How much will the vaccine cost and who will pay for it, AIIMS director Dr Randeep Guleria told NDTV, “Currently, the cost of the vaccine is supported by the government, so it will be something that is done as part of the government initiative.
Union Health Minister Dr. Harsh Vardhan himself arrived at GTB Hospital in Delhi this morning to take stock of the dry run. He said that the country has experience of vaccination. Vaccination has strict rules to follow. The Drug Controller General of India will take a decision on the vaccine soon.
Union Health Minister Dr. Harsh Vardhan said that the health workers have been given complete training in the country regarding dry runs. The Health Ministry has also formed a special team, which will closely monitor the entire process. Till now such a dry run was done in Punjab, Assam, Gujarat and Andhra Pradesh. In these four states, there were good results regarding the dry run. Keeping this in mind, now the government is going to start a dry run across the country.
Why the need for a dry run?
According to the Union Health Minister, Dr. Harsh Vardhan, through the dry run of vaccination, the country will examine the preparations for vaccination i.e. after the approval of the vaccine, the program of vaccination against corona in the country can be run very fast.
However, the final decision will be taken by the Drugs Controller General of India (DCGI). This means that soon after the approval of DGCI, the process of vaccination will start in the next 6-7 days. Let us tell you that the serum institute of India, Oxford and AstraZeneca have jointly developed the covisiled vaccine. After Britain and Argentina, India is the third country where Oxford has been recommended to allow the vaccine.