New Delhi: Drug 2-deoxy-D-glucose (2-DG) by the Institute of Nuclear Medicine and Allied Sciences (INMAS), Laboratory of Defense Research and Development Organization (DRDO) in collaboration with Dr. Reddy’s Laboratories (DRL), Hyderabad An anti-covid-19 therapeutic application has been developed. Clinical trial results have shown that this molecule helps in the rapid recovery of hospitalized patients and reduces dependence on oxygen delivery from outside. RT-PCR negative conversion was observed in the treatment of overdose covid patients with 2-DG. This drug will be very beneficial for people suffering from Covid-19.
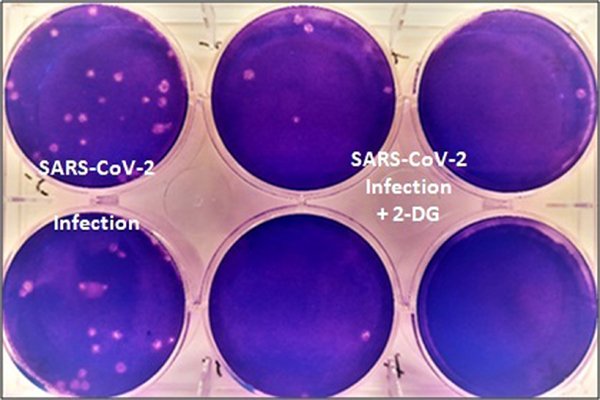
The Defense Research and Development Organization (DRDO) took the initiative to develop anti-covid therapeutic applications of 2-DG in connection with the call of Prime Minister Shri Narendra Modi to prepare against the epidemic. During the first wave of the pandemic in April 2020, INMAS-DRDO scientists conducted laboratory tests with the help of the Center for Cellular and Molecular Biology (CCMB), Hyderabad, and found that the drug worked effectively against the SARS-COV-2 virus. And inhibits viral growth. Based on these results, the Drugs Controller General of India (DCGI) Central Drugs Standard Control Organization (CDSCO) in May 2020 allowed phase-2 clinical trials of 2-DG in Covid-19 patients.
The Defense Research and Development Organization (DRDO) along with its industry partner DRL Hyderabad initiated clinical trials to test the safety and efficacy of the drug in Covid-19 patients. In Phase-II trials (including dose raging) conducted from May to October 2020, the drug Covid-19 was found to be safe in patients and showed significant improvement in their recovery. The second phase was conducted in six hospitals and Phase II B (dose raging) clinical trials were conducted in 11 hospitals across the country. In Phase-2, 110 patients were trialed.
The trends in efficacy showed patients treated with 2-DG exhibited faster symptomatic treatment than the standard of care (SOC) at various endpoints. During this treatment, a significant difference (2.5 days difference) was observed in the average time taken to normalize the parameters related to specific vital signs in the patient’s body compared to the standard of care (SOC).
DCGI allowed phase-3 clinical trials in November 2020, based on successful results. Phase-3 clinical trials were conducted on 220 patients between December 2020 to March 2021 in 27 Covid hospitals in Delhi, Uttar Pradesh, West Bengal, Gujarat, Rajasthan, Maharashtra, Andhra Pradesh, Telangana, Karnataka, and Tamil Nadu. Detailed data of the Phase III clinical trial were presented to DCGI. In the case of 2-DG, a significantly higher proportion of patients’ symptoms were seen, and by the third day, patients were relieved of supplemental oxygen dependence (42 percent, 31 percent) as compared to SOC indicating early relief from oxygen therapy/dependence.
A similar trend was observed in patients older than 65 years. On May 1, 2021, DCGI permitted the emergency use of this drug as adjuvant therapy in severe Covid-19 patients. Being a common molecule and analog of glucose, it can be easily produced and made available in greater quantities in the country.
This drug comes in the form of powder in a sachet which is dissolved in water. This virus accumulates in infected cells and inhibits the growth of the virus by inhibiting viral synthesis and energy production. Its selective accumulation in virus-infected cells makes this drug unmatched.
A large number of patients are currently experiencing severe oxygen dependence in the second Covid-19 wave released and need to be hospitalized. This drug is expected to save valuable lives due to the way the drug works in infected cells. This also reduces the number of days spent in the hospital for Covid-19 patients.
